On July 17, 2015, the Center for Developmental Cardiology published “E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1” in Nature Communications. Mitochondrial fragmentation plays an important role in the progression of cardiac diseases, such as myocardial infarction and heart failure. Mitochondria are the major ATP and reactive oxygen species production organelle in cardiomyocytes. Mitochondrial malfunction is tightly related to cardiovascular diseases and contributes to heart failure. Mitochondria function and morphology are closely associated. Mitochondria constantly undergo fission and fusion. Fission leads to the formation of small round mitochondria and promotes cell apoptosis, whereas fusion results in mitochondria elongation and plays a protective role in cardiomyocyte maintenance. Although emerging evidence has demonstrated that mitochondrial fission and fusion machinery are important in maintaining cardiac function, previous results have not indicated the transcriptional control of mitochondrial network regulators.
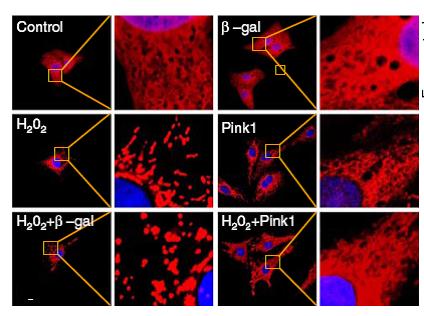
This research revealed that the interplay between E2F1, miR-421, and Pink1 could regulate mitochondrial morphology and cardiomyocyte cell death. Pink1 reduced mitochondrial fragmentation and protects cardiomyocytes from apoptosis. In contrast, miR-421 promoted cardiomyocyte mitochondrial fragmentation, apoptosis, and myocardial infarction by suppressing Pink1 translation. Finally, the transcription factor E2F1 activated miR-421 expression. Knocking down E2F1 suppressed mitochondrial fragmentation, apoptosis, and myocardial infarction by affecting miR-421 levels. Collectively, the authors identified that the E2F1/miR-421/Pink axis is a regulator of mitochondrial fragmentation and cardiomyocyte apoptosis and suggested potential therapeutic targets in the treatment of cardiac diseases.