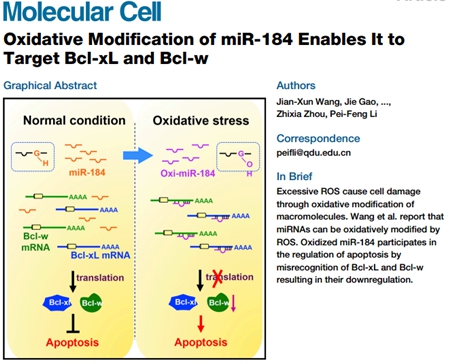
Recently, our Institute’s research results in the field of noncoding RNA and oxidative modification were published in Molecular Cell and Nature Communications. Excessive reactive oxygen species (ROS) can oxidize and modify biological macromolecules to make them function abnormally, thereby causing cell damage. Oxidative modification is related to cancer, aging, neurodegenerative diseases, and cardiovascular diseases. The article published in Molecular Cell reported that in our research, the oxidative-modified miR-184 could bind to the 3’ untranslated regions of Bcl-xL and Bcl-w, which were not its natural targets, and regulate their gene expression. We also confirmed that the mismatch between oxidative-modified miR-184 and Bcl-xL and Bcl-w was involved in initiating cell apoptosis at the cellular and animal levels. These results indicate that ROS participates in the regulation of cardiomyocyte apoptosis through oxidative modification of miRNA. The research was published in Molecular Cell, and the authors included Professor Wang Jianxun and others.
The article published in Nature Communications reported our findings that lncRNA could regulate the autophagy of cardiomyocytes. We named the newly discovered lncRNA an autophagy promoting factor (APF). The results of our research showed that miR-188-3p could inhibit autophagy-induced cardiomyocyte death or myocardial infarction by acting on autophagy-related gene ATG7. APF targeted miR-188-3p to regulate the level of ATG7 and thus regulated cardiomyocyte autophagy and myocardial infarction. This study revealed a new signal pathway composed of APF, miR-188-3p, and ATG7, which regulates the autophagy program of cardiomyocytes in the heart. The relevant factors in this pathway could provide potential targets and diagnostic tools for the development of new treatment strategies for myocardial infarction and heart failure. The research was published in Nature Communications, and the authors included Professor Wang Kun and others.